FAQs
Room 5 in the basement of the Biochemistry building
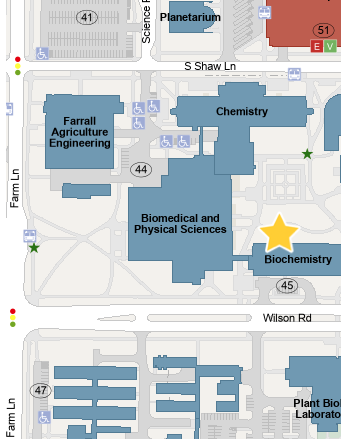
For MSU researchers, we use an online portal for sample submissions which can be found at https://michiganstate.sharepoint.com/sites/Proteomics. Contact us first to set up an account. Once you’ve created a new experiment in the system samples can be brought to room 5, Biochemistry and placed in our drop-box or you can hand them directly to us.
Off-campus customers can fill out a submission sheet and either email it back to us or include it in your shipment.
Samples must be isolated protein. This can be in the form of protein in lysis solution, precipitated protein pellets, protein bound to affinity resin or stained protein gel bands. We cannot accept live cells, tissues or biological fluids.
This depends. For simple protein identifications we generally need 10s of nanograms of purified protein. A simple metric is to run the protein on a 1D gel. If you can see a band by Coomassie or Silver staining this should be plenty of material to make an ID. More extensive characterization of a protein (ex. PTM identification, maximized sequence coverage) will require at least microgram quantities. Complex samples (ex. whole cell lysates) often require hundreds of micrograms. For samples in solution a concentration of at least 1ug/uL is recommended. If you’re not sure, we’d be happy to consult with you free of charge.
Unfortunately, there is no universal way to prepare samples for proteomic analysis but we can accept protein gel bands, protein solutions and precipitated protein pellets. Some experiment types are best served by a particular method so if you’re unsure we’d be happy to consult with you free of charge.
Samples which contain detergents, glycerol, urea (>1M) or salts (>200mM) can all interfere with analysis. However, these can often be removed or reduced to safe levels. Please indicate all buffer components when you submit samples or consult with us before preparation for best results.
For protein ID experiments replicates generally aren’t necessary but for more complex experiments, especially quantitative proteomics, replicates are strongly recommended. We suggest between an n=3 and n=5 in most cases. For targeted proteomics and affinity proteomics controls are strongly recommended to aid in data analysis. If you’re not sure we’re happy to consult free of charge.
- On-campus researchers will receive a link to download your results from our sharepoint page. You can log in at your convenience and download the results, raw data and metadata.
- Off-campus researchers will receive a link to dropbox where you can download your results and data. Projects too large for dropbox will instead receive a USB via FedEx containing the files.
Most results are returned as Scaffold files (proteomesoftware.com). The Scaffold viewer is freely available and offers a convenient tool for visualizing proteomics results. The tutorial videos on our website offers some quick introductions to using Scaffold and more extensive tutorials can be found on the Proteome Software website.
Once your project is complete you will receive a copy of all data files (raw data, metadata and results). Please save these files to a safe location. Our detailed data retention policy can be found here (https://rtsf.natsci.msu.edu/about/data-retention-policy.aspx)
We recommend guidelines established by the Association of Biomolecular Resource Facilities (ABRF) which can be found here:


